Experimental methods for determining crystal structure: Difference between revisions
mNo edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
==Scattering Methods== | ==Scattering Methods== | ||
There are various different techniques that are employed to probe the interior structure of the microscopic. Most make use of scattering described by Bragg's Law, bouncing various particles or waves off of the atoms that make up the target sample. From the distribution of spots on a film representing the points of constructive interference, the location of the atoms can be visually represented. | There are various different techniques that are employed to probe the interior structure of the microscopic. Most make use of scattering described by '''Bragg's Law''', bouncing various particles or waves off of the atoms that make up the target sample. From the distribution of spots on a film representing the points of constructive interference, the location of the atoms can be visually represented. | ||
Different waves or particles (as quantum wave-particle duality shows how all particles can be represented as waves, and conversely, waves can be represented as particles) will have different qualities that effect how they will scatter. Some of the common waves and particles used in scattering techniques are X-rays, electrons, and neutrons. | Different waves or particles (as quantum wave-particle duality shows how all particles can be represented as waves, and conversely, waves can be represented as particles) will have different qualities that effect how they will scatter. Some of the common waves and particles used in scattering techniques are X-rays, electrons, and neutrons. | ||
Revision as of 15:50, 19 April 2009
Scattering Methods
There are various different techniques that are employed to probe the interior structure of the microscopic. Most make use of scattering described by Bragg's Law, bouncing various particles or waves off of the atoms that make up the target sample. From the distribution of spots on a film representing the points of constructive interference, the location of the atoms can be visually represented.
Different waves or particles (as quantum wave-particle duality shows how all particles can be represented as waves, and conversely, waves can be represented as particles) will have different qualities that effect how they will scatter. Some of the common waves and particles used in scattering techniques are X-rays, electrons, and neutrons.
Bragg's Law
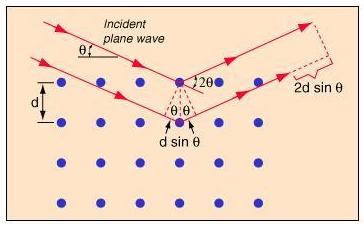
The core principle behind using waves scattered off atomic centers to obtain information about the internal structure of a crystal is that of Bragg's Law. It was developed by William Lawrence Bragg around the turn of the 20th century, along with his father William Henry Bragg. They shared a Nobel prize for this work in 1915.
Bragg's Law gives a relation between wavelength of the incident waves which are being used to scatter , the distance between the scattering centers, in this case, the atomic nuclei , and the angle between the waves and the plane formed by the atoms in the lattice .
Constructive interference occurs when the path difference between the two waves is an integer number of wavelengths, so they have the same phase at that point.
So, from the diagram to the right, constructive interference will be observed when,
where x is the length of the smaller leg of the triangle formed by the distance between atoms, and the perpendicular between waves. This distance that the second wave travels represents half of the total path difference between the two waves.
so,
When the incident waves hit the plane formed by the atomic centers, they reflect or transmit and can interfere either constructively or destructively. Where they constructively interfere, a film placed in the path of the waves will record a dot, and places with destructive interference record nothing. Doing this sort of scattering process from many different angles will give a 3-D pattern representing the location of atoms in the lattice.
X-ray scattering
X-ray scattering section.
Crystal sample size on the order of 100µm (1000 times thicker than crystals used in electron scattering).
Electron scattering
Because electrons do have a wave nature, they can also be scattered off atoms in a crystal lattice, this process is similar to X-ray scattering. Some differences are that
- Electrons used in this way have a smaller wavelength than X-rays, this allows for greater resolution.
- The thickness of the sample must be very small to be transparent with respect to the electrons, on the order of ~100nm.
Neutron diffraction
Similar to electron and X-ray diffraction, neutrons with wavelength ~0.1nm are used to scatter off atomic centers in a crystal to create a pattern from which its atomic structure can be derived.