Experimental methods for determining crystal structure: Difference between revisions
| Line 1: | Line 1: | ||
==Scattering Methods== | ==Scattering Methods== | ||
There are various different techniques that are employed to probe the interior structure of the microscopic. Most make use of scattering described by '''Bragg's Law''', bouncing various particles or waves off of the atoms that make up the target sample. This is an elastic scattering, where the wavelength of the wave after collision is the same as that of the incoming wave. Additionally, for such scattering to be effective in producing a diffraction pattern that can be analyzed, the waves, once scattered off atomic centers need to not scatter additional times. This is called secondary scattering, if the incident waves interact too strongly with the sample crystal, the secondary scattering caused will create a diffraction pattern that is difficult to analyze because the periodic pattern originally created from the corresponding periodic array of atoms will be altered dramatically. From the distribution of spots on a film representing the points of constructive interference, the location of the atoms can be visually represented. | There are various different techniques that are employed to probe the interior structure of the microscopic. Most make use of scattering described by '''Bragg's Law''', bouncing various particles or waves off of the atoms that make up the target sample. This is an elastic scattering, where the wavelength of the wave after collision is the same as that of the incoming wave. Additionally, for such scattering to be effective in producing a diffraction pattern that can be analyzed, the waves, once scattered off atomic centers need to not scatter additional times. | ||
This is called secondary scattering, if the incident waves interact too strongly with the sample crystal, the secondary scattering caused will create a diffraction pattern that is difficult to analyze because the periodic pattern originally created from the corresponding periodic array of atoms will be altered dramatically. From the distribution of spots on a film representing the points of constructive interference, the location of the atoms can be visually represented. | |||
Different waves or particles (as quantum wave-particle duality shows how all particles can be represented as waves, and conversely, waves can be represented as particles) will have different qualities that effect how they will scatter. Some of the common waves and particles used in scattering techniques are X-rays, electrons, and neutrons. | Different waves or particles (as quantum wave-particle duality shows how all particles can be represented as waves, and conversely, waves can be represented as particles) will have different qualities that effect how they will scatter. Some of the common waves and particles used in scattering techniques are X-rays, electrons, and neutrons. | ||
Revision as of 15:57, 20 April 2009
Scattering Methods
There are various different techniques that are employed to probe the interior structure of the microscopic. Most make use of scattering described by Bragg's Law, bouncing various particles or waves off of the atoms that make up the target sample. This is an elastic scattering, where the wavelength of the wave after collision is the same as that of the incoming wave. Additionally, for such scattering to be effective in producing a diffraction pattern that can be analyzed, the waves, once scattered off atomic centers need to not scatter additional times.
This is called secondary scattering, if the incident waves interact too strongly with the sample crystal, the secondary scattering caused will create a diffraction pattern that is difficult to analyze because the periodic pattern originally created from the corresponding periodic array of atoms will be altered dramatically. From the distribution of spots on a film representing the points of constructive interference, the location of the atoms can be visually represented.
Different waves or particles (as quantum wave-particle duality shows how all particles can be represented as waves, and conversely, waves can be represented as particles) will have different qualities that effect how they will scatter. Some of the common waves and particles used in scattering techniques are X-rays, electrons, and neutrons.
Bragg's Law
The core principle behind using waves scattered off atomic centers to obtain information about the internal structure of a crystal is that of Bragg's Law. It was developed by William Lawrence Bragg around the turn of the 20th century, along with his father William Henry Bragg. They shared a Nobel prize for this work in 1915.
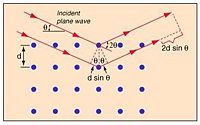
Bragg's Law gives a relation between wavelength of the incident waves which are being used to scatter , the distance between the scattering centers, in this case, the atomic nuclei , and the angle between the waves and the plane formed by the atoms in the lattice .
Constructive interference occurs when the path difference between the two waves is an integer number of wavelengths, so they have the same phase at that point.
So, from the diagram to the right, constructive interference will be observed when,
where x is the length of the smaller leg of the triangle formed by the distance between atoms, and the perpendicular between waves. This distance that the second wave travels represents half of the total path difference between the two waves.
so,
When the incident waves hit the plane formed by the atomic centers, they reflect or transmit and can interfere either constructively or destructively. In this way, the crystal lattice acts as a diffraction grating. Where the waves constructively interfere, a film placed in the path of the waves will record a dot, and places with destructive interference record nothing. Doing this sort of scattering process from many different, regular angles will give a 3-D pattern representing the location of atoms in the lattice.
X-ray scattering
In this method, electromagnetic waves, specifically monochromatic X-rays (X-rays of the same wavelength), are shot at a crystal sample that will act as a diffraction grating because of the periodic arrangement of atoms in crystal. The points recorded on some sort of film behind it will represent constructive interference, and by recording sufficiently many sets of reflections (the projected spots where constructive interference happens) from different angles, one can derive the arrangement of the lattice that created the reflections.
Electron scattering
Because of the wave nature of electrons, they can also be scattered off atoms in a crystal lattice. This process is similar to X-ray scattering. Some differences are that
- Electrons used in this way have a smaller wavelength than X-rays, this allows for greater resolution.
- The thickness of the sample must be very small to be transparent with respect to the electrons, on the order of ~100nm. This is because, unlike X-rays, electrons are much more likely to undergo secondary scattering. Decreasing the thickness of the sample prevents the scattered electrons from re-scattering and creating a diffraction pattern that is difficult to analyze.
The source of electrons that are scattered in this way is commonly a Transmission Electron Microscope (TEM), or Scanning Electron Microscope (SEM). A SEM generally uses a potential around 10kV, this voltage accelerates electrons used in this way to around 0.2c (c = speed of light, ). A TEM uses a larger potential of ~200kV, this higher voltage accelerates electrons to closer to 0.7c.
Electrons fired at a target from a SEM or TEM will have a much larger wavelength than X-ray used for X-ray diffraction, using the de Broglie wave
and actually are energetic enough to require a relativistic adjustment giving the form,
where is wavelength, is Planck's constant, is the mass of the electron, and is the potential used to accelerate the electrons.
Using this formula one finds the wavelength of the fired electrons to be ~10pm for the SEM and ~2pm for the TEM. Comparing this to the X-rays used in X-ray diffraction with wavelength of ~100pm (0.1nm), its easy to see this scattering method can provide a higher resolution.
Neutron diffraction
Similar to electron and X-ray diffraction, neutrons with wavelength ~0.1nm are used to scatter off atomic centers in a crystal to create a pattern from which its atomic structure can be derived.