Notes III
The branch of physics that studies the building blocks and interaction of atomic nuclei is called nuclear physics. The question of "What the Universe made of?" is one of the main interests to many of astrophysicists and cosmologists today. To determine the terrestrial abundance of elements in the Universe the basic tools of nuclear physics are employed.
Nuclear reactions not only explain the bulk solar-system abundance distribution, but are also indispensable for explaining the observed chemical composition of individual starts.
Atomic Nuclei
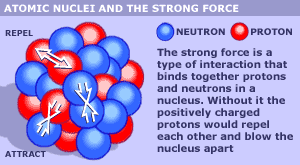
The nucleus of an atom is a very dense region that constitutes of protons and neutrons. The protons and the neutrons are also called as nucleons. The force that holds the quarks and gluons to make nucleons is known as a strong force and the residual effect of this strong force is called the nuclear force that acts between nucleons.
Generally the atomic nucleus is denoted by , where denotes the number of protons in the nucleus and is dubbed as atomic number, denotes the number of neutrons, denotes the number of nucleons and is dubbed as mass number, and denotes the element symbol. An individual nuclear species are known as nuclides. There are three types of nuclides: isotopes (the same , but different and hence different ), isobars (the same but different and ) and isotones (the same , but different and hence different ).
Nuclides can be represented in a 2-dimensional diagram called Chart of the nuclides. Where the is on the vertical axis and is on the horizontal axis. The shaded squares are for stable nuclides and open squares correspond to unstable nuclides.
Definitions for Abundances
The particle abundance is determined by
where the sum is over all the isotopes and is the particles number density. The so-called relative abundance is found through the formula
We also introduce the mass fraction
Denoting
we can write
The mean molecular weight is determined by
The electron abundance
is the ratio of protons to nucleons in the sample. Obviously, the electron number density is then found by
Solar System Abundances
The solar system is commonly believed to have formed from the collapse of a gaseous nebula. And this pre-solar nebula was thought to have an almost homogeneous abundance distribution of elements. There are three methods of probing the elemental abundances in the presolar nebula: The study of Materials on earth, the solar spectra, and unfractionated meteorites.
Earth materials
Examining materials on earth that have not undergone chemical fractionation gives a small glimpse at what elements were present in the presolar nebula.(Most isotopic compositions have not undergone chemical fractionation and are the main source of information for this.)
Solar Spectra
The Sun formed directly from the presolar nebula, and the study of it's spectrum can shed light on its composition. Of the outer layers, the photosphere is believed to be the most accurate representation of the elemental composition of the primordial solar system.
Meteorites
The analysis of presolar grains in a specific class of meteorites, called CI carbonaceous chondrites, can be perhaps the most precise measurement of this elemental abundance. These carbonaceous chondrites never fractionated because they did not experience high pressures or temperatures. There are only five known CI carbonaceous chondrites and they make up only an extremely small fraction of the meteorite.