Liquid Crystal: Difference between revisions
Jump to navigation
Jump to search
MatthewHoza (talk | contribs) |
MatthewHoza (talk | contribs) |
||
| Line 21: | Line 21: | ||
'''Methods to Measure Orientational Order, <math>S</math>''' | '''Methods to Measure Orientational Order, <math>S</math>''' | ||
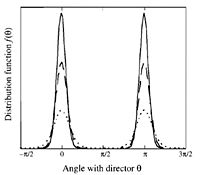
[[IMAGE:DistFunc.jpg|right|thumb|200px|Distribution function of the order parameter, S. | [[IMAGE:DistFunc.jpg|right|thumb|200px|Distribution function of the order parameter, S. Zero represents complete disorder, while one represents complete order.]] | ||
* The direction of orientation is shown by the ''director''. Each molecule makes an angle with respect to the director. Measure the angle and average over all the molecules. The more orientational order present the closer the average angle will be to zero. | * The direction of orientation is shown by the ''director''. Each molecule makes an angle with respect to the director. Measure the angle and average over all the molecules. The more orientational order present the closer the average angle will be to zero. | ||
Revision as of 18:30, 13 April 2009
Brief Synopsis
History
- Many scientists had already observed this phenomenon of liquid crystals prior to its "discovery", however the Austrian botanist Friedrich Reinitzer is more often then not given credit for its discovery.
- Friedrich Reinitzer
Description
- Phase of matter "between" solid and liquid
- Cloudy liquid between solid and liquid phase.
- Liquid Crystal flows and will take the shape of its container , however the cloudiness suggests it differs from liquids.
- Solids possess both positional order (molecules are constrained to occupy certain positions) and orientational order (the manner in which the molecules are oriented with respect to one another ?only nearest neighbor?). When a solid melts to a liquid both types of order are completely lost. However, when a solid melts to a liquid crystal only the positional order is completely lost.
- Molecules are free to move about as in a liquid, however they on average spend more time pointing along the direction of orientation.
Methods to Measure Orientational Order,
- The direction of orientation is shown by the director. Each molecule makes an angle with respect to the director. Measure the angle and average over all the molecules. The more orientational order present the closer the average angle will be to zero.
- Measure the angles with respect to the director and then plug them into the function:
where is the fraction of molecules in a solid angle which are oriented at an angle of to the director.
Now perfect oriental orientation will have an average of 1 and no orientational order will have an average of 0. The average of this function is called the order parameter.
- Insert Chart Collings pg. 11
- Can also take the average angle of one molecule over a certain time, instead of looking at different molecules. This only works under the assumption that all molecules undergo random motion. This assumption has never been contradicted experimentally.
- Latent Heat shows that liquid crystal is closer to a liquid than it is to a solid.
- Some molecules are more likely to form into a liquid crystal than others. There are 3 properties that make a molecule a good candidate to become liquid crystal(Collings pg.12):
(1) Elongated in shape
(2) Rigid in the center
(3) Flexible on the ends
- Thermotropic : Order Determined by temperature
- Lyotropic
Phases
- Isotropic: Identical in all directions
- The different phases have different amounts of positional and orientational order
- Nematic: No positional order; long-range orientation order
- Smectic: Positionally ordered in 1D and orientational order
- Columnar (discotic): Positional order in 2D and orientational order
Applications
- Many common liquids are liquid crystals (i.e. soap)
Displays
- Televisions
Thermometer
- Mood Ring
Future
Takaki: 3D Display
Electronic Paper
Sources
Chien, Liquid Crystal Materials, Devices, and Applications IX
Collins, Liquid Crystals
Jones, Soft Condensed Matter