PHZ3400 Phase Transition: Difference between revisions
Jump to navigation
Jump to search
NickDeguzman (talk | contribs) |
StevenDolly (talk | contribs) |
||
| Line 3: | Line 3: | ||
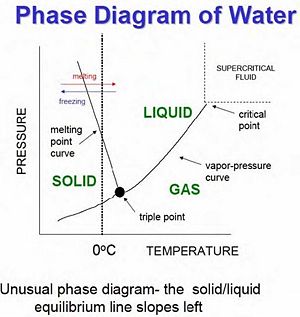
==Gas-Liquid-Solid Phase Diagram== | ==Gas-Liquid-Solid Phase Diagram== | ||
[[Image:PhaseDiagram.jpeg|thumb|right|300px|Phase Diagram of Water]] | |||
==Phase Separation and Nucleation== | ==Phase Separation and Nucleation== | ||
Revision as of 12:32, 28 January 2009
Phases of Matter
Matter can exist in many phases. The phases most common are solids, liquids, and gases. In Physics, a phase can be described as a region of space in which all physical properties of a material remain constant, or uniform. Having consistent physical properties and chemical uniformity allow one to distinguish between the various phases, or states of matter.
Gas-Liquid-Solid Phase Diagram
Phase Separation and Nucleation
This we'll cover on Friday, Jan. 30
Why Ice Floats? Consequences.
Van der Waals Equation
The Van der Waals Equation is an equation of state, that is a modification of the ideal gas law:
Johannes van der Waals proposed two modifications to the ideal gas law:
Van der Waals Equation:
where
is pressure
is temperature
is the number of particles
is volume
and are constants dependent on the material