PHZ3400 Phase Transition: Difference between revisions
MatthewHoza (talk | contribs) |
|||
| Line 3: | Line 3: | ||
Soft matter is very complicated. What is remarkable about soft matter is its ability to put itself into complicated arrangements, all without outside assistance. This is known as self-assembling. There are two ways to classify self-assembled structures: at equilibrium or non-equilibrium, which are structures that are a result of a phase change. Soft matter often shows rather complex equilibrium phases. | Soft matter is very complicated. What is remarkable about soft matter is its ability to put itself into complicated arrangements, all without outside assistance. This is known as self-assembling. There are two ways to classify self-assembled structures: at equilibrium or non-equilibrium, which are structures that are a result of a phase change. Soft matter often shows rather complex equilibrium phases. | ||
After a phase transition, what is usually the result is a self-assembled structure at non-equilibrium. These phase transitions usually result in a less ordered state, which can be shown through liquids and solids; liquids are less ordered than solids. The characterize these phase transitions, they are ordered, which is based on the nature of the transition. A first-order phase transition is where order parameters (such as temperature and energy) changes discontinuously at the phase transition. This differs from a second-order phase transition where order parameters remain continuous. This uniformity of a second-order phase transition is well represented in the change from a liquid to a gas at its critical point. | After a phase transition, what is usually the result is a self-assembled structure at non-equilibrium. These phase transitions usually result in a less ordered state, which can be shown through liquids and solids; liquids are less ordered than solids. The characterize these phase transitions, they are ordered, which is based on the nature of the transition. A first-order phase transition is where order parameters (such as temperature and energy) changes discontinuously at the phase transition. This differs from a second-order phase transition where order parameters remain continuous. This uniformity of a second-order phase transition is well represented in the change from a liquid to a gas at its critical point. | ||
A first-order phase transition is where order parameters (such as temperature and energy) changes discontinuously at the phase transition. | |||
This kind of phase transition represents crossing a boundary between phases on a phase diagram. This type of transition differs from a second-order phase transition where order parameters change continuously. This uniformity of a second-order phase transition is well represented in the change from a liquid to a gas around its critical point. Instead of directly crossing the boundary from liquid to gas, the liquid can be pressurized to exceed the pressure of the critical point, then heated to exceed the temperature of the critical point, and finally depressurized and cooled to a gas in a continuous change of phase. | |||
==Gas-Liquid-Solid Phase Diagram== | ==Gas-Liquid-Solid Phase Diagram== | ||
Revision as of 02:30, 6 February 2009
Phases of Matter
Matter can exist in many phases. The phases most common are solids, liquids, and gases. In Physics, a phase can be described as a region of space in which all physical properties of a material remain constant, or uniform. Having consistent physical properties and chemical uniformity allow one to distinguish between the various phases, or states of matter. Soft matter is very complicated. What is remarkable about soft matter is its ability to put itself into complicated arrangements, all without outside assistance. This is known as self-assembling. There are two ways to classify self-assembled structures: at equilibrium or non-equilibrium, which are structures that are a result of a phase change. Soft matter often shows rather complex equilibrium phases. After a phase transition, what is usually the result is a self-assembled structure at non-equilibrium. These phase transitions usually result in a less ordered state, which can be shown through liquids and solids; liquids are less ordered than solids. The characterize these phase transitions, they are ordered, which is based on the nature of the transition. A first-order phase transition is where order parameters (such as temperature and energy) changes discontinuously at the phase transition. This differs from a second-order phase transition where order parameters remain continuous. This uniformity of a second-order phase transition is well represented in the change from a liquid to a gas at its critical point.
A first-order phase transition is where order parameters (such as temperature and energy) changes discontinuously at the phase transition. This kind of phase transition represents crossing a boundary between phases on a phase diagram. This type of transition differs from a second-order phase transition where order parameters change continuously. This uniformity of a second-order phase transition is well represented in the change from a liquid to a gas around its critical point. Instead of directly crossing the boundary from liquid to gas, the liquid can be pressurized to exceed the pressure of the critical point, then heated to exceed the temperature of the critical point, and finally depressurized and cooled to a gas in a continuous change of phase.
Gas-Liquid-Solid Phase Diagram
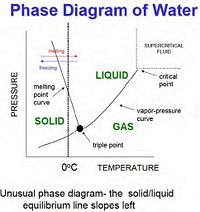
When studying phase transitions, it is important to be able to read and understand a phase diagram. A phase diagram is a pressure vs. temperature graph of an element or compound that shows which phase we can expect it to be in (see right for example). The lines represent the boundaries between phases, and at any point on these lines we can expect two (or even three) phases to exist simultaneously in equilibrium. In the area between curves only one phase of matter exist. There are two important points on phase diagrams which should be mentioned:
Triple Point - At this specific temperature and corresponding pressure, the three phases of matter can exist simultaneously in equilibrium. This means that evaporation, condensation, freezing, melting, fusion, and sublimation are happening at once.
Critical Point - At this point in temperature and pressure (and at any point above this), the boundary between liquid and gas doesn't exist any more. Here a gas cannot be distinguished from a liquid, and the heat of vaporization is zero. However, you can still have a smooth transition between gas and liquid by going up and around the critical point (as seen on the phase diagram) by passing through the supercritical fluid region.
Phase Separation and Nucleation
When a fluid is in the co-existence dome of a phase diagram, phase separation occurs. This happens because the density of the fluid is in between that of a liquid and that of a gas, so both phases exist at once (hence the name co-existence dome). Phase separation occurs because of a continuous change in composition, which is done by a process known as spinodal decomposition. In spinodal decomposition, any small changes made in composition are amplified as a result of the free energy being lowered. This also is a result of the system being unstable.
Nucleation: is the process where gas begins to condense to form liquid droplets, but both co-exist at the same time. Usually there will be a global phase separation, where the gas and liquid separate out completely. But when dealing with charged particles charge neutrality forbids this, so instead nano-scale phase separation occurs where the two phases do not completely separate, but droplets form. The energy domain of the wall of these droplets (E) is directly proportional to the surface area (S) of these droplets by some constant :
Looking at this equation we can now see why a sphere is the most energy efficient shape for the droplets to be in. A sphere will have the smallest surface area (as opposed to a cube or other 3D shapes). It then follows that:
This relates the energy E to the radius R of the droplet. We can see from this equation that as R is small the first term dominates, and as R becomes very large the second term dominates. By taking the derivative of this equation and setting it equal to zero we can find the maximum of this function, which physically represents the critical droplet size of nucleation.
Vlad: You need to include ALL the derivations I presented in class, when preparing these lecture notes!!! Please do it promptly
Why Ice Floats? Consequences.
An object will float if it is less dense than the fluid that it has been placed in. For an object of mass m to float, it must displace mass m of liquid.
Water reaches its maximum density at 4 degrees C and then continues to freeze. When it freezes, the solid ice is less dense than the liquid water. Usually, the liquid state of a material is less dense than the solid. The difference in the case of water is hydrogen bonding.
{{#ev:youtube|gmjLXrMaFTg}}
The water molecule is composed of two hydrogen atoms and one oxygen atom held together by covalent bonds. The molecules have a weak attraction to each other in the form of a hydrogen bond. This occurs between the positively charged hydrogen atoms in the molecule and the negatively charged oxygen atoms in another molecule. When water freezes into a solid, the hydrogen bonds adjust such that the negatively oxygen atom in each molecule are far apart. This creates a lattice that is less dense than the liquid form.
Van der Waals Equation
Vlad: You need to include ALL the derivations I presented in class, when preparing these lecture notes!!! Please do it promptly
We know that each atom or a molecule in a liquid has a very steep repulsive potential at short distances, due to the Pauli exclusion principle. Thus a volume comparable to the atomic radius is essentially excluded to other particles. This considerably limits the volume of space accessible to thermal motion of every atom or molecule.
Johannes van der Waals incorporated this into his theory. To do this he made two modifications to the ideal gas law,
His first modification was to restrict the volume of a molecule by introducing a minimum volume, . The constant then represents the minimum volume occupied by a molecule, when it's "touching" all its neighbors. This modification gives us:
Van der Waals second modification to the ideal gas law was to add the term . This term is the pressure due to the total potential energy, which accounts for the short-range attractive forces between molecules when they are not touching. This second modification gives us Van der Waals Equation:
is pressure
is temperature
is the number of particles
is volume of the container containing the fluid
is the measure of attraction between particles
is the volume excluded by a mole of particles.
An alternate form of the Van der Waals Equation:
where
Behavior Near Critical Point
We can derive the behavior of Van der Waals Equation near the critical point to discover how a fluid behaves near the critical point. As we increase to some critical temperature, we find that
Using these critical values we can scale Van der Waals Equation to put it in its universal form.
In this form isotherms corresponding to the given scaled temperature would collapse on a single curve. This predicts the universal number:
Experimental evidence shows that many different fluids have a ratio of 0.3, which is in agreement with the theory.
Sources
"Soft Condensed Matter", Richard A.L. Jones
"An Introduction to Thermal Physics", Daniel V. Schroeder