Phase transitions in biological systems
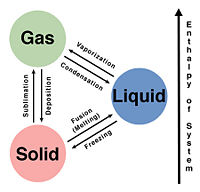
Phase transitions are present everywhere. Even within something as complex as the human body or plants, phase transitions are still present throughout processes which these systems use to survive. Critical phenomena such as second-order phase transitions are hard to find in certain biological systems however first-order phase transitions are easily present. Trying to analyze a biological system to define that phase transitions that occur within the system can sometimes be difficult but they are there.
Biological Materials as Soft Condensed Matter?
Why yes! Many biological materials can be considered to be soft condensed matter. Believe it or not, but muscles and blood exhibit properties which allows them to be classified as soft condensed matter. Blood can be classified as a colloid, which is a chemical substance consisting of a mix of various things. The various things that make up a colloid are not dissolved in it, but rather are distributive evenly within the substance. The neat thing about colloids is that they're able to exist in gaseous, liquid, and solids forms.
The Human Body as a Biological System
The human body is a very complex biological system with many organ systems within itself that allow bodily process to maintain life. Within these systems, various phase transitions do occur. Many of these phase transitions are necessary in order for things to work.
Respiratory System
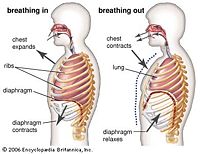
Respiration itself requires many phase transitions. It begins simply with the inhalation of O2 gas which enters the lungs. Inhalation is done by stimulation of pulmonary muscles which allows the diaphragm to contract, allowing oxygen to enter the lungs. Oxygen gas gets absorbed into the bloodstream through little compartments that make up the lungs known as alveoli by way of diffusion. The oxygen gas binds to a solid protein known as hemoglobin, and is then carried throughout the body in blood, which in can be considered a liquid. In term of phase transitioning, the oxygen gas diffuses into liquid blood and remains there by way of binding to a solid protein. At the same time, carbon dioxide gas (CO2)is also moving through the blood stream where it will diffuse into the lungs and out of the body by exhalation.
Digestive System
For a human, digestion begins in the mouth. There are various salivary enzymes that break down solid foods such as starches. Also food is broken down by chewing, which not only makes swallowing easier, but it also increases the surface area capacity for digestion by breaking down solid particles into smaller pieces. When solid food particles enter the stomach, many acids and enzymes are released which aid in the breakdown and digestion of food. The release of a neurotransmitter known as acetylcholine, or Ach, triggers the release of more gastric juices. Protein in the stomach bonds to hydrogen ions in order to lower the pH to strong acid levels. These pH levels allow the enzyme pepsin to work optimally in order to denature proteins. Proteins can be denatured by heating them up to high temperatures however if these high temperatures were reached within a biological system, it is possible that the high temperature can cause the denaturing of proteins vital towards the survival of that biological system. This is the case with second-order phase transitions and why it is typically not found within a biological system like a human body. The denaturing process in the stomach is different because instead of using high temperatures to break polypeptide bonds in proteins, the strong acidic activity by pepsin actually reduces the structure of these polypeptide bonds. What is meant by structure is the primary, secondary, tertiary, and quaternary structure of proteins. These typically involve the sequences of amino acids that make up the protein as well as chemical bonding and atomic composition. The by-product of a lot of these reactions in the stomach is gases. Typically, acid-base reactions such as these occurring in the stomach release carbon dioxide and water as by-products so for a phase transition, we see there that the enzymatic activity of the stomach’s gastric juices in combination with solid food particles releases gas. In the stomach, liquids such as alcohols are absorbed directly into the circulatory system. They are able to pass through because they are extremely small molecules and are able to pass through the membrane of the stomach. However, the solid food does not stay solid for long. Over time, solid food begins to turn into a semi fluid mass known as chyme. Chyme is partially digested food and begins to move past the stomach into the small intestine. Once again, more digestion is occurring and nutrients begin to be absorbed by blood. What is eventually leftover is wastes and they begin to build up and move into the large intestine where water is extracted from waste and eventually expelled out of the system as a solid.
Circulatory System
Transportation
Nervous System
Various signaling pathways
Reproductive System
The fertilization of an egg by sperm is irreversible.
Homeostasis
Homeostasis is the regulation of a system in order to maintain stable and constant conditions. Within a biological system such as a human body, many factors contribute to homeostasis, many of which involve some kind of phase transitioning. Regulating temperature within the human body is an intrinsic process that involves many things. One way of cooling the human body is evaporation by means of sweating. The question is, how does sweating cool the body? During hard workouts or when in warm climates, people tend to sweat, usually resulting in some cooling sensation. What is actually happening is that the sweat is evaporating from the skin. The transition from liquid to gas of the sweat on your skin results in a loss of energy, or a net outward transfer of heat.
Plants as a Biological System
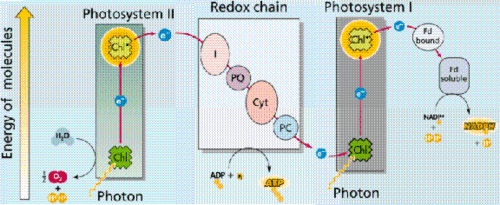
Photosynthesis
Biological Thermodynamics
Biological Thermodynamics combines the laws of thermodynamics with biology in order to explain or possibly quantify changes in energy from reactions. Many organ systems within the human body, (if not all of them), require some energy in order to function. In a plant, photosynthesis converts sunlight to energy usable by the plant so that it may synthesize sugars out of carbon dioxide gas and water. There has to be some sort of method to quantify the energy required in these reactions.
Sources
1. “Digestion”, 15APR09, <http://en.wikipedia.org/wiki/Digestion>
2. Fox, Stuart Ira, “Human Physiology”, 10th Edition, McGraw-Hill Companies, 2008
3. “MIT Department of Physics: Biophysics: Biological and Medicine”, 15APR09,< http://web.mit.edu/physics/research/areasofresearch/atomic_cm_plasma/biophysics/biophysics.html>
4. “Nervous system”, 15APR09, <http://en.wikipedia.org/wiki/Nervous_system>
5. “Phase Changes”, 15APR09, < http://hyperphysics.phy-astr.gsu.edu/hbase/thermo/phase.html>
6. “Phase transition”, 15APR09, <http://en.wikipedia.org/wiki/Phase_transition>
7. “Photosynthesis”, 15APR09, <http://www.emc.maricopa.edu/faculty/farabee/BIOBK/BioBookPS.html>
8. “Photosynthesis”, 15APR09, <http://en.wikipedia.org/wiki/Photosynthesis>
9. “Soft Condensed Matter: where physics meets biology”, 15APR09,< http://physicsworld.com/cws/article/print/169>
10. “Types of Photosynthesis”, <http://wc.pima.edu/~bfiero/tucsonecology/plants/plants_photosynthesis.htm>
11. "Biological Thermodynamics", <http://en.wikipedia.org/wiki/Biological_thermodynamics>
12. "Soft matter", <http://en.wikipedia.org/wiki/Soft_matter>
13. "Colloid", <http://en.wikipedia.org/wiki/Colloids>