Nucleation, droplets, and the physics of clouds
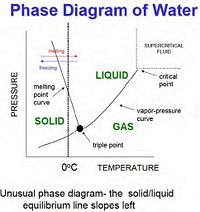
Nucleation is the process which results in the formation of a particle (or nuclei) of a different thermodynamic phase from a solution, liquid, or vapor. This process is very important, as almost every first-order phase transition proceeds from nucleation. Some second order processes, such as ferromagnetic reorientation and crystallization, are also caused by nucleation. There will be some basic equations and definitions for second-order nucleation, but since the physics of clouds deals with first order phase transitions (namely condensation), most of this project will be devoted to nucleation in first order phase transitions. This process occurs all throughout science and nature and has several applications, both harmless and dangerous, from the delicate formation of clouds to the explosion caused by placing Mentos in Coke. Nucleation is also very important in the development of semiconductors. In nature this process occurs at an interface, such as the surface of a container which holds the fluid or the surface of a particle of dust which is in the solution. This is referred to as heterogeneous nucleation and happens much more often than homogeneous nucleation, which occurs in completely pure liquids. This type of nucleation requires activation by supercooling or superheating the fluid.
{{#ev:youtube|g4kBNBEJKD8}} A more dangerous application of nucleation!
Phase Transitions
Nucleation Definition & Derivation