Liquid Crystal
Discovered more than 100 years ago, liquid crystals still remain an area of research today.
History
Many scientists had already observed this phenomenon of liquid crystals prior to its "discovery", however the Austrian botanist Friedrich Reinitzer is more often then not given credit for its discovery. While experimenting with cholesterol benzoate in 1888, Reinitzer noted that it the substance had two melting points. The first melting point occurred at 145.5°C, where it melted to a cloudy liquid phase, and 178.5°C, where it melted to a clear liquid phase. He also observed some strange color phenomena as the temperature changed.
Reinitzer then sent some of his samples to a German physicist, Otto Lehmann, who had a state of the art polarized microscopic, which he outfitted with heating stage so he could precisely control the temperature of his specimens. Lehmann first described Reinitzer's sample as floating crystals and crystalline fluids, but after more experimentation became convinced that the cloudy liquid was a uniform phase all its own. This led him to coin the term liquid crystal to describe this new phase of matter.
In the early 1900s, many advances were made in the understanding liquid crystals. In 1922, French physicist, Georges Freidel, gave a detailed description of different liquid crystal phases, coining many terms that are still used today. Freidel also explored the orienting effect of an electric field on liquid crystals.
After World War II interest in liquid crystals began to wane. Many scientists believed that all of the important problems regarding liquid crystals had been solved. However, in the 1960s scientists began to re-examine liquid crystals and continued the progress of the pre-war era. It was discovered that liquid crystalline substances were extremely sensitive to very small changes in temperature. Researchers at RCA found that with an electrical voltage applied, a thin layer of liquid crystal was capable of switching from cloudy to clear.
Description
Overview
Liquid crystals are a phase of matter "between" the solid phase and the liquid phase. Liquid crystal is "between" that of solid and liquid in that it displays characteristics common in both.
Solids possess both positional order, where molecules are constrained to occupy only certain positions, and orientaitional order, where each molecule is oriented with respect to one another. When a material transitions to a liquid both the positional and orientational order are lost. In the liquid crystal phase, molecules, or mesogen, display a wide range of positional and orientational orders, leading to several subphases. These subphases are defined by the amount of order present in the liquid crystal.
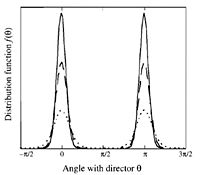
Methods to Measure Orientational Order
To quantitatively describe the amount of orientational order present in a subphase of liquid crystal the average direction of the mesogen, is measured with respect to a direction of orientation known as the director. Each molecule makes an angle, with respect to the director. This angle is then measured and averaged over all molecules.
The more orientational order present the closer the average will be to zero.
To create a more standard method of measuring orientational order we introduce the order parameter . Where once again we take the angle with respect to the director, however this time we use the function,
Now integrating over the solid angle,
where is the fraction of molecules in a solid angle which are oriented at an angle of to the director.
Now perfect oriental orientation will have an average of 1 and no orientational order will have an average of 0.
Phases
- Isotropic: Identical in all directions
- The different phases have different amounts of positional and orientational order
- Nematic: No positional order; long-range orientation order
- Smectic: Positionally ordered in 1D and orientational order
- Columnar (discotic): Positional order in 2D and orientational order
- which gives this phase characteristics unique to both the solid and liquid phase.
- Cloudy liquid between solid and liquid phase.
- Liquid Crystal flows and will take the shape of its container , however the cloudiness suggests it differs from liquids.
- Solids possess both positional order (molecules are constrained to occupy certain positions) and orientational order (the manner in which the molecules are oriented with respect to one another ?only nearest neighbor?). When a solid melts to a liquid both types of order are completely lost. However, when a solid melts to a liquid crystal only the positional order is completely lost.
- Molecules are free to move about as in a liquid, however they on average spend more time pointing along the direction of orientation.
- Insert Chart Collings pg. 11
- Can also take the average angle of one molecule over a certain time, instead of looking at different molecules. This only works under the assumption that all molecules undergo random motion. This assumption has never been contradicted experimentally.
- Latent Heat shows that liquid crystal is closer to a liquid than it is to a solid.
- Some molecules are more likely to form into a liquid crystal than others. There are 3 properties that make a molecule a good candidate to become liquid crystal(Collings pg.12):
(1) Elongated in shape
(2) Rigid in the center
(3) Flexible on the ends
- Thermotropic : Order Determined by temperature
- Lyotropic
Applications
- Many common liquids are liquid crystals (i.e. soap)
Displays
- Televisions
Thermometer
- Mood Ring
Continuing Research
Takaki: 3D Display
A Novel 3D Display Using an Array of LCD Panels (2003), Yasuhiro Takaki, Tokyo University
Insert Figure 6
- No need for 3D Glasses
- No restriction on observing position
- No fatigue
Electronic Paper
References
Chien, Liquid Crystal Materials, Devices, and Applications IX
Collins, Liquid Crystals
Jones, Soft Condensed Matter