Metallic Glass
A metallic glass, also known as an amorphous metal or glassy metal, is a metallic material that lacks a crystalline structure. Metals typically have a highly-ordered crystalline structure, whereas a metallic glass is amorphous in nature, much like a glass. Various methods are used to fix the metal's structure in an amorphous state, and the result is a material which possesses a number of novel properties with promising future applications.
How it's made
All metallic glasses are produced by cooling a metal alloy quickly enough so that crystals do not have time to form. Just how this is achieved has evolved since these materials were first discovered.
Rapid cooling
The first metallic glass was produced in 1959 by W. Klement (Jr.), Willens and Pol Duwez at Caltech. It was a 75/25 gold/silicon alloy that was solidified by cooling it from the liquid state at an extremely high rate of roughly one million Kelvin per second. This rapid cooling process forced the material into an amorphous state by not allowing the metal atoms enough time to order themselves into a crystal lattice. An unfortunate limitation of this process was that metallic glasses could only be formed as extremely thin samples, generally under a hundred microns thick, as heat needed to be removed quickly in order to frustrate crystallization of the material. This rapid cooling technique remained the only viable method for producing metallic glasses for nearly three decades, and with the limitations of the method, few applications could be realized.
Although the production method didn't much change, the necessary rate of cooling did see a dramatic decrease over the years. In 1969, a 77.5/16.5/6 palladium/silicon/copper alloy was developed that could be cooled at a much slower rate on the order of several hundred Kelvin per second. Various other alloy formulations allowed continued progress toward still lower minimum cooling rates.
Crystal frustration
It wasn't until the early 1990s that theoretical advances led to a significant reduction in cooling rates. Building on the theories of Harvard researcher David Turnbull, by 1990 Akahisa Inoue's group at Japan's Tohoku University had succeeded in producing samples of metallic glass up to a quarter of an inch thick. Dubbed bulk metallic glasses for their hefty achievable thickness, these revolutionary materials were made possible by composing the alloy from three or more elements whose atomic radii differ by at least twelve percent. These alloys solidify via something known as the "confusion principle", whereby the the significant variation in atomic sizes frustrates the formation of a crystal lattice, thus "confusing" the atoms as to what crystalline structure to settle into. This slows down the crystallization process to such a high degree that such alloys can be made to settle into an amorphous structure at a much slower rate of cooling than traditional metallic glasses, thus allowing much greater thicknesses to be achieved.
In 1992, William Johnson's group at Caltech developed the first of a series of metallic glass alloys known as Vitreloy, the first of which consisted of a 41.2/22.5/13.8/12.5/10 zirconium/beryllium/titanium/copper/nickel blend. This material cooled so slowly that it could be cast to thicknesses of over an inch.
Structure
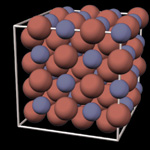
Typical metals have a highly-ordered crystalline structure consisting of repeating unit cells. An important consequence of this regular structure is that crystals have cleavage planes, so forces applied along these planes can lead much easier to deformation of the crystal. Metals are also frequently polycrystalline, meaning that they are not composed of a single crystal, but rather multiple crystals with different orientations. As an ordinary metal cools, crystallization begins randomly at different locations within the material, producing numerous individual crystals known as crystallites. As these crystallites continue to grow, eventually they run up against other crystallites. The surfaces along which these growing crystals collide are known as grain boundaries, and these are an important defect present in metals and many other crystalline materials. The presence of grain boundaries in a metal leads to a number of generally undesirable effects, including decreased electrical and thermal conductivity and reduced strength, as well as facilitating the onset of corrosion.
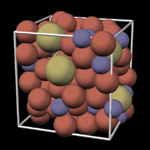
In contrast, metallic glasses have an amorphous structure,
(how they get there: rapid cooling, differing atom sizes, etc)
(no grain boundaries -> consequences)
(other stuff)
Properties
Strength
Elasticity
Injection moldable
(etc)
Shear and fatigue
http://www.sciencedaily.com/releases/2009/03/090324091211.htm http://blogs.physicstoday.org/update/2009/03/confining-cracks-in-metallic-g.html http://www.msnbc.msn.com/id/29738048/
The problem
The solution
Uses
Today
Amorphous metal transformers
The future
Blades, golf clubs, etc.
Coatings
(etc)
References
Lemley, Brad. Glassy Metal, Discover, April 2004