Phy5670: Difference between revisions
JorgeBarreda (talk | contribs) |
JorgeBarreda (talk | contribs) |
||
| Line 20: | Line 20: | ||
The laws that govern the universe present different kind of symmetries as translational and rotational symmetries, which tell us about the homogenity and isotropy of the space. Also we can talk about inversion and time-reversal symmetry. We can expect that basic symmetries are compatible with the matter at high temperatures, but not necessarly when it cools down as a crystal where the atoms are in fixed positions and the translational symmetry is no loger valid. | The laws that govern the universe present different kind of symmetries as translational and rotational symmetries, which tell us about the homogenity and isotropy of the space. Also we can talk about inversion and time-reversal symmetry. We can expect that basic symmetries are compatible with the matter at high temperatures, but not necessarly when it cools down as a crystal where the atoms are in fixed positions and the translational symmetry is no loger valid. | ||
Following this idea we can talk about a broken symmetry when we pass from a liquid to a solid, or also when we pass from paramagnet to a ferromagnet. Obiously in a change of phase the system can no longer be described with the same equation of state and a new description is necessary. | Following this idea we can talk about a broken symmetry when we pass from a liquid to a solid, or also when we pass from paramagnet to a ferromagnet. Obiously in a change of phase the system can no longer be described with the same equation of state and a new description is necessary. | ||
We need to remark that boken symmetry is not the only way to have a change in the qualitatived behavior of the matter, but these cases are relatively rare. For example when a water pass from gas to liquid water, there is not broken symmetry. | We need to remark that boken symmetry is not the only way to have a change in the qualitatived behavior of the matter, but these cases are relatively rare. For example when a water pass from gas to liquid water, there is not broken symmetry. | ||
==== <tt>"Why?"</tt> broken symmetry ==== | ==== <tt>"Why?"</tt> broken symmetry ==== | ||
Revision as of 15:29, 1 October 2010
Welcome to the Quantum Many Body Physics PHY5670 Fall2010
PHY5670 is a one semester graduate level course. Its aim is to introduce basic concepts, and logical framework, of this vast and developing discipline: broken symmetry and adiabatic continuity. Theoretical techniques, such as coherent state path integrals and diagrammatic perturbation expansions, will be used to emphasize these deeper underlying concepts, as well as to provide practical means of calculations. Few illustrative physical systems and quantum many-body models will also be studied.
The key component of the course is the collaborative student contribution to the course Wiki-textbook. Each team of students is responsible for BOTH writing the assigned chapter AND editing chapters of others.
Team assignments: Fall 2010 student teams
Outline of the course:
Conceptual basis of many body physics
Broken symmetry
Our experience shows us, then, that as matter cools down it usually no longer retains the full symmetry of the basic laws of quantum mechanics which it undoubtedly obeys; our task here is to understand that the questions we must ask are "Why", "In what sense", and "What are the consequences?" P.W. Anderson (Basic Notions of Condensed Matter Physics)
The laws that govern the universe present different kind of symmetries as translational and rotational symmetries, which tell us about the homogenity and isotropy of the space. Also we can talk about inversion and time-reversal symmetry. We can expect that basic symmetries are compatible with the matter at high temperatures, but not necessarly when it cools down as a crystal where the atoms are in fixed positions and the translational symmetry is no loger valid.
Following this idea we can talk about a broken symmetry when we pass from a liquid to a solid, or also when we pass from paramagnet to a ferromagnet. Obiously in a change of phase the system can no longer be described with the same equation of state and a new description is necessary.
We need to remark that boken symmetry is not the only way to have a change in the qualitatived behavior of the matter, but these cases are relatively rare. For example when a water pass from gas to liquid water, there is not broken symmetry.
"Why?" broken symmetry
Under surprisingly general circumstances the lowest energy state of a system does not have the total symmetry group of its Hamiltonian, and so in the absence of thermal fluctuations the system assumes an unsymmetrical state. P.W. Anderson (Basic Notions of Condensed Matter Physics)
Examples: Wigner crystallization and He-3
At low density, electrons in the jellium model crystalize into a bcc Wigner crystal. At high density (and sufficiently finite temperature) they behave as Fermi gas/liquid with all the invariance properties of the ordinary Boltzman gas, which this system is when .
If we compress a high enough density Fermi liquid with short range repulsive interactions, approximately as , it will form a regular solid.
The essential phenomenon is either case is that the lowest state of a potential energy of interaction between the particles -- for example, a pair interaction
-- must occur for either a unique relative configuration of all particles and all translations and rotations,
or, in artificial cases, perhaps for a highly restricted subset of all configurations. P.W. Anderson (Basic Notions of Condensed Matter Physics)
It is then clear that in any situation where the potential energy dominates kinetic energy and entropy, as in the two cases mentioned, a system of particles obeying a simple potential will take up a regular lattice structure. P.W. Anderson (Basic Notions of Condensed Matter Physics)
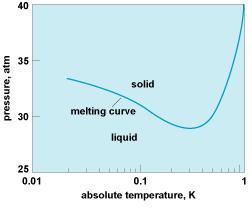
The less symmetric state tends to be one lower in temperature, simply because the more symmetric one is usually a distribution of thermal fluctuations among all the available values of the order parameter . But this order of phases is not a general rule; He3, for instance, violates it because the solid has a greater nuclear paramagnetic entropy than liquid, and at low temperatures the melting curve has a negative slope. So in this temperature regime, the solid is the high-temperature phase and the liquid is the low-temperature phase. P.W. Anderson (Basic Notions of Condensed Matter Physics)
Role played by the Thermodynamic limit
"What are the consequences?" of broken symmetry
- discreteness of phase transitions, and the resulting failure of continuation: disjointness of physical phases
- development of collective excitations
- generalized rigidity
- defect structures: dissipation and topological considerations