Double Slit Experiment
Bullet
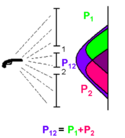
Imagine a gun which is shooting bullets randomly toward a wall with two slits in it separated by a distance, . The slits are about the size of a bullet. A histogram of the bullet's location after it passes through the two slits is plotted. If slit 2 is closed, but the slit 1 is open, then the green peak is observed which is given by the distribution function . Similarly, if the slit 1 is closed, but he slit 2 is open, the pink peak is observed which is given by the distribution function . When both slits are open, (purple) is observed. This agrees with the classical view, where the bullet is the particle and is simply a sum of and . The bullets do not follow purely linear trajectories because they are allowed to hit the edges of the slits they pass through and be deflected. It is because the bullets can be deflected that the result of this experiment is a probability distribution rather than the bullets going to just the two locations that are along straight line trajectories from the gun through the slits.
The equation describing the probability of the bullet arrival if both of the slit are open is therefore
Classical Waves
As waves are passed through the double slit, they are diffracted so that the waves emerge from the slit as circular waves. This effect can only occur when the size of the slits is comparable to the wavelength. The intensities and of the waves, which are proportional to the squares of their amplitudes, are observed when only slit 1 and slit 2 are open, respectively. These intensities are similar to the histograms for the bullets in the previous demonstration. However, an interference pattern of intensity is observed when both slits are opened. This is due to constructive (peaks) and destructive (troughs) interference of the two waves.
Hot Tungsten Wire (thermal emission of electrons)
A high current is passed through a tungsten wire, resulting in electrons being emitted from the wire which then enter the double slits one at a time, arriving in the same manner as the bullet arrives from the gun. However, after plotting a histogram of the locations where the electron landed, it looks like for the double slit wave experiment. This shows that electrons exhibit both the wave-like and the particle-like character. The probability distribution of the electron's landing on the screen thus exhibits the interference patterns. It is the laws obeyed by these probability "amplitudes" that quantum mechanics describes.